how to find the initial rate of reaction
3. Differential Rate Laws
Methods for determining order of reaction
This section looks at how concentration affects reaction charge per unit. You lot will acquire how to determine the order of a reaction. Reactions are often categorized into get-go, 2d, third order, etc. and this information is useful for showing the mathematical relationship between concentrations and rates.
Rate laws may be written using two different but related perspectives:
- A differential rate law
- An integrated charge per unit police
In this section, we will focus on differential rate laws, integrated rate laws will be discussed in particular in the adjacent page. Before we brainstorm, download a flowchart hither that will help you navigate this lesson:
Download flowchart >>
Rate of reaction
The charge per unit of reaction studies how fast a reaction takes identify – which is concerned with speed. The study relates the change in concentration (reactant or production) over time elapsed.
rate = [concentration] vs time
Society of reaction
The order of reaction studies how a reaction's rate is affected by the concentration of each reactant in the reaction. The studies relate to the change in concentration (reactants) to the charge per unit of reaction.
lodge = rate vs [concentration]
Differential rate law equation
In calculating lodge of reaction, we are only interested in the concentration of the reactants and Not the products.
A very elementary reaction A + B → C + D , where A and B are the reactants and C and D are the products, we can discover the order of reaction past using the charge per unit equation:
rate = thousand[A]ᵐ[B]ⁿ
Note that (k and n) are not coefficients of the equation, they are the reaction order of each reactant.
Information technology is important to understand that rate constabulary or rate society of a reaction can simply be experimentally obtained.
Determining order of reaction
A simple example experimental data is given below:
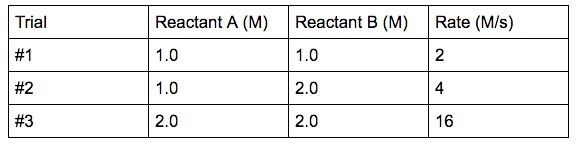
In the experiment, if we vary the concentration of one reactant at a fourth dimension, we will exist able to found how the charge per unit changes with respect to each reactant.
Reactant A is held abiding, the concentration of reactant B is doubled. What is the event on the charge per unit? (See trial #1 & #2)
Equate the ratio
We tin equate the ratio of (change in concentration) to (ratio of change in rate).
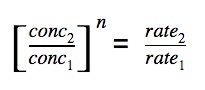
The society of reaction is simply n.
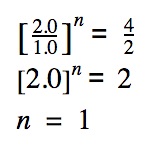
In this case, the order of reaction with respect to reactant B is ane.
The rate club is calculated for 1 reactant only. Now, we need to calculate the rate club with respect to the other reactant.
The order of reaction with respect to reactant A is calculated equally follows:
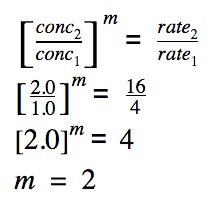
The overall order of reaction
The overall order of reaction is the sum of private orders of reaction of the reactants.
![]()
Overall lodge of reaction = three
The rate law for the reaction:
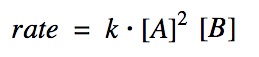
Zeroth order of reaction
The zeroth order reaction does non depend on the concentration of the reactants.
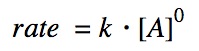
Recall that thou is a constant that is dependent on temperature.
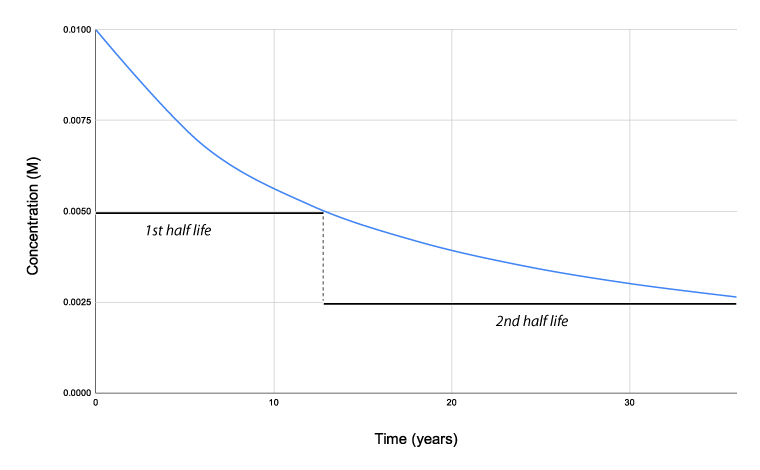
Half-life
One-half life is the time it takes for one-half of the reactant to reach half of its concentration.
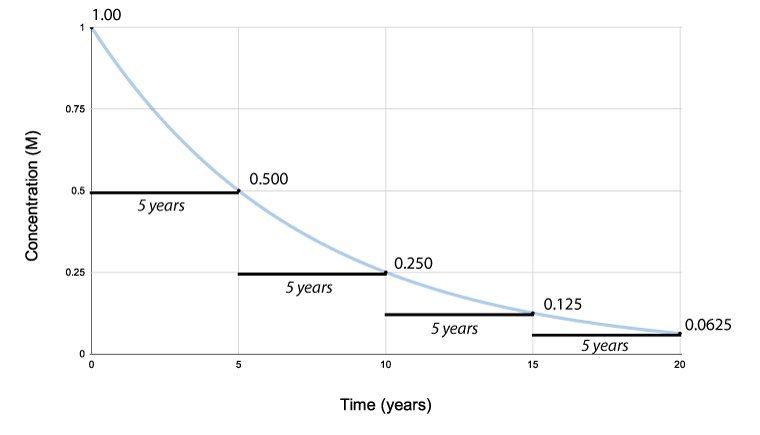
One-half life for a 1st order reaction is a abiding. A good case of half-life of 1st order reaction is radioactive decay. The concentration of the reactant decreases past a abiding with each half–lifeand is independent of the concentration of the reactant. The above instance shows that the concentration of the reactant decreases by one-half the amount every five years interval.
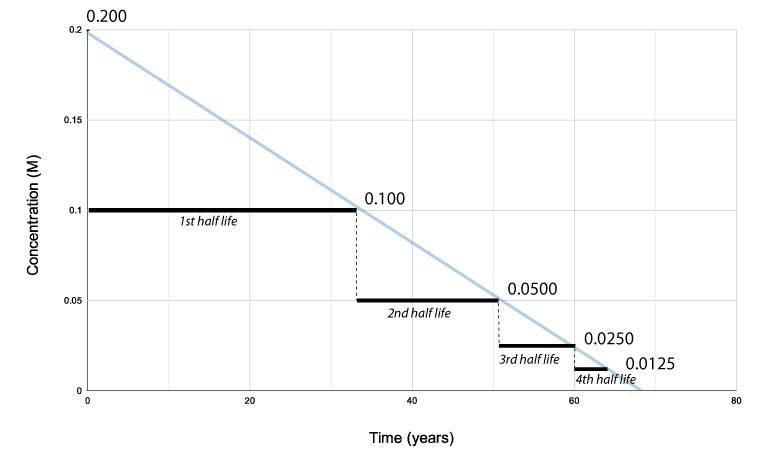
The half-life of a second-lodge reaction decreases as the concentration increases.
The half-life of a zeroth-order reaction decreases as the concentration decreases.
Notation: Zeroth-society and second-order reactions practice not have a constant half-life. Merely a 1st order reaction has a abiding half-life.
Summary of charge per unit law

Zeroth order : rate does not depend on reactants
First order : rate depends on ane 1st order reactant
2d social club : charge per unit depends on two 1st club reactants or ane 2nd lodge reactant
Source: https://viziscience.com/ap-chemistry-resources/chemical-kinetics/order-of-reaction/
Posted by: gravescolmilluke.blogspot.com

0 Response to "how to find the initial rate of reaction"
Post a Comment